950
Views & Citations10
Likes & Shares
The study aim was to evaluate the antioxidant potential of Biofield
Energy Healing Treatment (The Trivedi Effect®) on a novel
proprietary test formulation in male Sprague
Dawley rats. The test formulation was divided into two parts. One part was
denoted as the control without any Biofield Energy Treatment, while the other
part was defined as the Biofield Energy Treated test formulation. Additionally,
three groups of animals were also received Biofield Energy Healing Treatment per se (day 15). The test formulation
was evaluated for antioxidant enzymes, hematology, biochemistry, organ weight
and histopathology analysis. The antioxidant results showed that the
glutathione (GSH) level was significantly increased by 26.95%, 33.66%, 71.59%,
28.50% and 86.15% in the Biofield Energy Treated test formulation (G5),
Biofield Energy Treatment per se to
animals at day 15 (G6), Biofield Energy Treated test formulation from day 15
(G7), Biofield Energy Treatment per se
to animals with the Biofield Energy Treated test formulation from day 15 (G8)
and Biofield Energy Treatment per se
to animals with untreated test formulation (G9) groups, respectively as
compared to the disease control (G2) group. Antioxidant enzyme like glutathione
peroxidase (GPx) level was significantly increased by 22.12%, 37.88%, 48.71%
and 21.18% in G5, G7, G8 and G9 groups, respectively as compared to the G2. The
level of myeloperoxidase (MPO) was decreased by 15.70%, 13.41%, 21.56% and
11.80% in G5, G6, G7 and G8 groups, respectively as compared to the G2.
Hematology profile showed an improvement of total leukocyte count (TLC) level
by 62.5%, 55.05%, 63.03% and 16.75% in the G6, G7, G8 and G9 groups,
respectively as compared with the G2 group. Lipid profile data showed a
significant reduction of triglycerides and very low density lipoprotein (VLDL)
levels by 51.39% and 51.56%, respectively in the G8 group as compared with the
G2 group. Hepatic biomarkers analysis showed decreased serum glutamate
oxaloacetate transaminase (SGOT) level by 24.84%, 57.89% and 17.43% in the G5,
G6 and G8 groups, respectively as compared with the G2 group. Further, the
level of serum glutamate pyruvate transaminase (SGPT) was significantly
decreased by 47.70% and 19.30% in the G6 and G8 groups, respectively compared
with the G2 group. However, relative organ weight (%) and histopathology data suggested
that there were no treatment-related changes in any group, which was found to
be safe without any side-effect during the course of the experiment. These data
suggested that the Biofield Energy Treated test formulation and The Trivedi
Effect®-Consciousness Energy Healing Treatment per se can be used for improving the antioxidant enzymes levels
that might be useful against many autoimmune and inflammatory diseases, stress
management and prevention and act as anti-aging therapy by improving overall
body’s detoxification process.
Keywords: Consciousness
energy healing, The Trivedi effect®, Immunomodulation, Nanocurcumin,
Antioxidant, Hematology, Biochemistry
INTRODUCTION
Today, herbal based remedies
are accepted worldwide and are back into the prominence. The use of such
Complementary and Alternative Medicines (CAM) has become increasingly popular
in the developed world [1,2]. For complementary therapies, plants or plant
based constituents are always the key source of treatment strategy in various
medicinal systems. In recent years, combination of herbal product (polyherbal)
or single herbs has been used as curative
substance in order
to improve the
health
MATERIALS AND METHODS
Requirements
Iron sulfate, copper
chloride, cholecalciferol, streptozotocin, cyclophosphamide and sodium
carboxymethyl cellulose were obtained from Sigma Chemical Co. (St. Louis, MO).
Nanocurcumin was purchased from Sanat Products Ltd., India. Quercetin dihydrate
was procured from Central Drug House Pvt. Ltd., India. Magnesium (II) gluconate
and zinc chloride were obtained from TCI, Japan. Sodium selenate and ascorbic
acid were procured from Alfa Aesar, USA. All other chemicals used in this study
were analytical grade available in India.
Laboratory animals
Randomly breed male Sprague Dawley (SD) rats with body
weight ranges between 200-280 g were used in this experiment. The animals were
purchased from M/s. Vivo Bio Tech Ltd., Hyderabad, India. Standard rodent diet
was procured from M/s. Golden feeds, Mehrauli, New Delhi, India and provided ad libitum to all the groups of animals
during the experiment under controlled conditions with a temperature of 22 ±
3°C, humidity of 30% to 70% and a 12 h light/12 h dark cycle. The animals were
acclimatized for the period of 5 days prior to the experiment and all were
accessed once daily for clinical signs, behaviors, morbidity and mortality. All
the procedures were in strict accordance with the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of Health. The
approval of the Institutional Animal Ethics Committee was obtained prior to
carrying out the animal experiment.
Study design
The animals were
randomized and grouped according to their body weight. A total of nine groups
(G) were included, i.e., Group 1 (G1) was served as a normal control (i.e.,
vehicle control) and G2 was served as a disease control; both the groups were
received 0.5% Na-CMC, while G3 group animals received quercetin dihydrate (100
mg/kg; p.o.) as positive control. G4 group animals received untreated test
formulation and G5 group animals received Biofield Energy Treated test
formulation at a dose of 624.12 mg/kg. Similarly, G6 group animals received
Biofield Energy Treatment (15 days) per
se, G7 animals received Biofield Energy Treated test formulation (15 days);
G8 group defined as Biofield Energy Treated animals + Biofield Energy Treated
test formulation (15 days) and G9 group denoted as Biofield Energy Treatment per se to animals plus untreated test
formulation.
Biofield energy treatment strategies
The test formulation
was divided into two parts. First part of each ingredient was considered as
control, where no Biofield Energy Treatment was provided. Second part of each
ingredient and three groups (G6, G8 and G9) of animals were received Biofield
Energy Treatment (also known as The Trivedi Effect®-Consciousness
Energy Healing) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar
Trivedi under laboratory conditions for ~3 min. The energy transmission was
done without touching the samples and animals. Similarly, the control samples
were subjected to “sham” healer under the same laboratory conditions for ~3 min.
The “sham” healer did not aware about the Biofield Energy Treatment. After
that, the Biofield Energy Treated samples were kept in the similar sealed
condition and used as per the study plan. The Biofield Energy Treated animals
were also is taken back to the experimental room for further proceedings.
Experimental procedure
Five days after the
acclimatization, animals were randomized and grouped based on body weight.
After 15 days pre-study period the G6 group was received vehicle; while G7 and
G8 groups were received the test formulation. The animals were fasted for 15-18
h and were injected with streptozotocin (STZ 45 mg/kg, i.p. single dose). After
1 week of post STZ injection, basal glucose levels (tail cut method) were
measured for confirmation of diabetes (Day 1). The animals were treated with
the test formulation/vehicle/positive control daily for up to 56 days. The body
weight was recorded daily throughout the experimental period. On day 56, 50% of
animal population was kept for overnight fasting and day 57 animals were bled
and the samples subjected for hematology, biochemistry and electrolytes
analysis. After bleeding, animals were humanely sacrificed to collect organ,
i.e., liver. A portion of liver samples was weighed and transferred to the
prescribed homogenizing buffer. The collected liver samples were homogenized and
stored in -80°C for the estimation of various antioxidant parameters (GSH, GPx
and MPO) using commercially available kit.
Antioxidant assay using ELISA method
Estimation of antioxidants - GSH and GPx: For the estimation
of GSH, the liver sample was used, which is based on the reduction of 5, 5
dithiobis (2-nitrobenzoic acid) (DTNB) with reduced glutathione (GSH) to
produce a yellow compound. The reduced chromogen is directly proportional to
the GSH concentration and its absorbance was measured at 405 nm by using a
commercial kit (Item No: 703002, Cayman Chemicals) [28]. Liver tissues (GPx)
enzyme activity was measured as IU/g tissue by the reaction between glutathione
remaining after the action of GPx and 5, 5-dithiobis-(2-nitrobenzoic acid) to
form a complex that absorbs maximally at 412 nm. The sample absorbance was
measured at 405 nm by using a commercial kit (Item No: 703102, Cayman
Chemicals) [29].
Anti-inflammatory marker, MPO: For MPO estimation,
liver tissue (5%w/v) was homogenized in 0.5% hexadecyl trimethyl ammonium
bromide (HTAB, Sigma-Aldrich, Co., St. Louis, MO, USA) with 50 mM potassium
phosphate buffer, pH 6. The rest of the steps were performed as per in-house
standard protocol. In addition, the homogenate was used for the estimation of
myeloperoxidase (MPO) using Elisa kit (Cat No: k11-0575, Kinesisdx) through the
colorimetric method as per manufacturer recommended standard procedure [30].
Measurement of hematology parameters
For the estimation of
hematology, blood was withdrew from the retro-orbital plexus by capillary tubes
and the hematology parameters such as differential leukocyte count (DLC), total
leukocyte count (TLC), and lymphocyte, neutrophil, eosinophil and monocyte were
evaluated using Hematology analyzer (Abbott Model-CD-3700) [31].
Measurement of hepatic enzymes and lipid profile
Serum biochemistry
parameters viz. high density
lipoprotein (HDL), total cholesterol (TC), low density lipoprotein (LDL),
triglycerides (TG), very low density lipoprotein (VLDL), serum glutamic oxaloacetic
transaminase (SGOT), alkaline phosphatase (ALP), serum glutamate-pyruvate
transaminase (SGPT), creatine kinase-myocardial band (CK-MB), total protein
(TP), total bilirubin (TB), albumin (A), globulin (G) and albumin/globulin
ratio (A/G) were analyzed in the test formulations [31].
Clinical sign and symptoms
The animal clinical
sign and symptoms were evaluated once daily throughout the experiment in
accordance with in-house protocol with few modification [32]. Animals found in
a moribund or even enduring signs of severe distress were humanely euthanized.
Abnormal findings were noted with the time of onset and disappearance.
Measurement of organ weight and histopathology
After completion of
the experiment, rats were dissected and the whole liver, kidneys, hearts,
spleens, lungs and testis were excised, freed of fat, blotted with clean tissue
paper, and then weighed. The organ to body weight ratio was determined by
comparing the weight of each organ with the final body weight of each rat.
Defined samples were placed in 10% neutral buffered formalin for
histopathological examination.
STATISTICAL ANALYSIS
Each experiment was
carried out in eight independent assays and was represented as mean ± standard
error of mean (SEM). Student’s t-test
was used to compare two groups to judge the statistical significance. For
multiple group comparison, one-way analysis of variance (ANOVA) was used
followed by post-hoc analysis using Dunnett's test. Statistically significant
values were set at the level of p ≤
0.05.
RESULTS AND DISCUSSION
Effect of the test formulation on antioxidant parameters
Antioxidant activity
of the novel test formulation was studied using ELISA method by estimating
various enzymes such as antioxidants viz.
GPx and GSH; and acute inflammatory marker viz.
MPO. Liver homogenate of rat in various groups were used for the estimation of
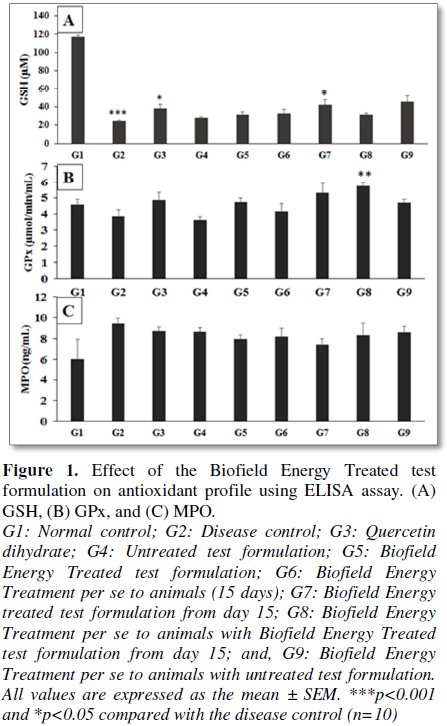
antioxidants enzymes and results are presented in Figure 1. The administration of novel test formulation and Biofield
Energy Healing Treatment per se
results in significant decrease in the content of enzymatic antioxidants (GPx)
and non-enzymatic antioxidants (GSH) in cyclophosphamide (G2) group (Figure 1A). However, GSH was
significantly increased by 26.95%, 33.66%, 71.59%, 28.50% and 86.15% in the G5,
G6, G7, G8 and G9 groups, respectively as compared to the diseases control
group G2. In addition, GPx level was increased by 22.12%, 7.06%, 37.88%, 48.71%
and 21.18% in the G5, G6, G7, G8 and G9 groups, respectively as compared to the
diseases control group G2 (Figure 1B).
Acute inflammatory marker, MPO concentration was significantly decreased in the
test formulation groups in comparison with the G2 group. The level of MPO was
decreased by 15.70%, 13.41%, 21.56%, 11.80% and 8.46% in the G5, G6, G7, G8 and
G9 groups, respectively as compared to the diseases control group G2 (Figure 1C). However, the level of MPO
was decreased after Biofield Energy Healing treatment by 8.31%, 5.80%, 14.68%
and 4.06% in the G5, G6, G7 and G8 groups, respectively as compared to the
untreated test formulation group (G4). Antioxidant activity is considered as
one of the vital property of any formulation or nutraceuticals. However, the
high concentration of free radicals is very much accountable for abundant
inflammatory infections [33]. Overall, the experimental data suggested that the
novel test formulation has the significant antioxidant activity, which might
help to minimize the inflammatory responses against wide range of inflammatory
disease conditions.
Analysis of hematological parameters
The effects of the
Biofield Energy Treated and untreated test formulations along with Biofield
Energy Treatment per se on animal
serum lipid profile are presented in Table
2. Among the estimated parameters; significant decreased level of total
cholesterol (87.80 ± 5.53 mg/dL), triglycerides (66.95 ± 20.02 mg/dL) and VLDL
(13.38 ± 4.00 mg/dL) were found in the Biofield Treated formulation (G5) as
compared with the disease control (G2) group. The level of total cholesterol,
triglycerides and VLDL was significantly decreased by 7.14%, 42.70% and 42.69%,
respectively in G5 group as compared with the G2 group. However, triglycerides
and VLDL levels were significantly reduced by 51.39% and 51.56%, respectively
in the G8 group, respectively as compared with the G2 group. With respect to
serum lipids; there was a reduction in the VLDL levels in the Biofield Energy
Treated test formulation and Biofield Energy Treated per se group as compared with the disease control and untreated
test formulation groups. Scientific literature suggested that the all the
active constituents in the test formulation were reported with the beneficial
effect on blood lipid profile. Individual ingredients such as nanocurcumin,
minerals and vitamins have been reported for significant decreased level of
triglycerides, serum cholesterol, LDL and VLDL levels. Major component of the
formulation, nanocurcumin has been found to have beneficial role in improving
the lipid profile [36]. Minerals such as selenium were reported to have
beneficial role in lowering the serum total cholesterol and LDL along with
improved humoral immunity [37]. Likewise, zinc and magnesium were found to have
improved lipid profile such as decreased total cholesterol, triglycerides and
LDL level, while increased HDL levels [38,39]. Overall, the results suggested
that the Biofield Energy Treated test formulation groups and Biofield Energy
Treatment per se showed significantly
improved lipid profile as compared with the untreated test formulation, which
can be used as better hypocholesterolemia agent.
Measurement of hepatic
biomarkers
The effect of proprietary novel formulation on hepatic parameters is
presented in Table 3. The data
suggested that the disease control (G2) group significant changed the level of
hepatic biomarkers, which were standardized by quercetin dihydrate along with
the Biofield Energy Treated Test formulation and Biofield Energy Treatment per se group.
The level of SGOT was reduced by 24.84%, 57.89% and 17.43% in the G5,
G6 and G8 groups, respectively as compared with the G2 group. However, SGPT
level was decreased by 47.70% and 19.30% in G6 and G8 groups, respectively as
compared with G2 group. The level of ALP was decreased by 2.77%, 6.79% and
3.41% in the G5, G7 and G8 groups, respectively as compared with the G2. CK-MB
level was reduced by 9.88% and 3.43% in G6 and G7 groups, respectively as
compared with untreated test formulation (G4) group. The alteration in hepatic
enzymes directly reflects the severity to the hepatocellular damage. An
increase in liver enzymes in blood reflects the extent of damage, which will
affect the liver function [40]. Scientific literature suggests that the
constituents of test formulation such as nanocurcumin reported to have
significant hepatic protection effect [41]. Similarly, minerals and vitamins
present in the test formulation have significance liver protection action that
helps to prevent the liver disease by stabilizing the membrane activity and
hepatic biomarkers [42]. Therefore, it is concluded that Biofield Energy
Healing Treatment per se and Biofield
Energy Treated test formulation have significant capacity to protect the liver
enzymes and can be used against many liver disorders.
Analysis of animal weight
parameters
After treatment, all the animals in different groups were studied for
their organ weight, which was compared with their initial body weight during
experimental periods (Table 4).
Overall, the experimental analysis data showed the final weights of tested
organs showed no significant change in various groups from G1 to G9. The values
were presented and compiled as organ to body weight ratio (expressed as
relative organ weight in percentage). However, no significant change was
observed in the tested organ weight throughout the experiment such as the organ
weight of liver, lungs, kidneys, brain, heart, eyes, spleen, pancreas, thymus,
small intestine, large intestine, testis, prostrate, epididymis and vas deference
with respect to the normal control and disease control group throughout the
exposure period. In addition, the body weight of all the animals in various
groups has been altered during the study period but not significant, which
suggested that the Biofield Energy Treated test formulation and Biofield Energy
Treatment per se (day 15) were found
to be safe and non-toxic during the exposure period.
Histopathological analysis was performed in all the groups after
treatment and analysis suggested that no treatment-related changes were
observed as compared with the normal control groups (Figure 2). Overall, the tested organ weight of all the animals was
represented as relative organ weight (%) which suggested no significant change.
Literature suggest that histopathological abnormalities such as swelling,
atrophy or hypertrophy data can be used to understand the pathological
conditions, which is the useful index to test any formulation for toxicity
assay [43,44]. After treatment with any test formulation, if body weight and
organ weight changed significantly then it suggested toxicity of the product.
Atrophy refers to the decrease in organ weight, while increase in body or organ
weight defined as hypertrophy in animals after exposure to the test
formulation. However, data suggest that there was no significant change in all
the treatment groups, which represent non-toxic and safe nature of the Biofield
Treated test formulation and Biofield Energy Healing Treatment per se throughout the exposure period.
CONCLUSION
Among tested
antioxidants, GSH level was significantly increased by 26.95%, 33.66%, 71.59%,
28.50% and 86.15% in the G5, G6, G7, G8 and G9 group, respectively, as compared
to the diseases control group G2. GPx level was increased by 22.12%, 7.06%,
37.88%, 48.71% and 21.18% in the G5, G6, G7, G8 and G9 groups, respectively as
compared to the diseases control group G2. However, anti-inflammatory marker
MPO was decreased by 15.70%, 13.41%, 21.56%, 11.80% and 8.46% in the G5, G6,
G7, G8 and G9 groups, respectively, as compared to the diseases control group
G2. Hematology data after treatment with the Biofield Energy Treated test
formulation showed a significant increase in the TLC level by 62.5%, 55.05%,
63.03% and 16.75% in the G6, G7, G8 and G9 groups, respectively as compared
with the G2 group. Lipid profile data showed that the total cholesterol,
triglycerides and VLDL were significantly decreased by 7.14%, 42.70% and
42.69%, respectively, in the G5 group as compared with the G2 group. Similarly,
total cholesterol, triglycerides and VLDL levels were also reduced by 3.4%,
51.39% and 51.56% in the G8 group, respectively, as compared with the G2 group.
Hepatic biomarker analysis revealed that SGOT level was reduced by 24.84%,
57.89% and 17.43% in G5, G6 and G8 groups, respectively, as compared with the
G2 group. On the other hand, SGPT level was significantly decreased by 47.70%
and 19.30% in the G6 and G8 groups, respectively as compared with G2 group. In
addition, ALP level was decreased by 2.77%, 6.79% and 3.41% in the G5, G7 and
G8 groups, respectively, as compared with the G2 group. However, no
treatment-related changes were observed in any experimental treated group with
respect to the relative organ weight (%) values in the Biofield Energy Treated
test formulation and Biofield Energy Treatment per se groups throughout the experiment. Overall, the data
suggested that The Trivedi Effect®-Consciousness Energy Healing
Treatment enhanced the test formulation’s antioxidant action. Thus, the
Biofield Energy Treated test formulation and Biofield Energy Treatment per se in male SD rats showed
significant antioxidant activity along with improved blood profile. Further, it
can be used as a Complementary and Alternative Medicine (CAM) with a safe therapeutic
index for various autoimmune disorders such as Lupus, Systemic Lupus
Fibromyalgia, Erythematous, Hashimoto Thyroiditis, Addison Disease, Celiac
Disease (gluten-sensitive enteropathy), Dermatomyositis, Multiple Sclerosis,
Graves’ Disease, Pernicious Anemia, Myasthenia Gravis, Scleroderma, Aplastic
Anemia, Psoriasis, Reactive Arthritis, Rheumatoid Arthritis, Sjogren Syndrome,
Type 1 Diabetes, Vasculitis, Crohn’s Disease, Chronic, Fatigue Syndrome
Vitiligo and Alopecia Areata, as well as inflammatory disorders such as
Irritable Bowel Syndrome (IBS), Asthma, Ulcerative Colitis, Alzheimer’s
Disease, Parkinson’s Disease, Atherosclerosis, Dermatitis, Hepatitis and
Diverticulitis. Further, the Biofield Energy Healing Treated test formulation
can also be used in the prevention of immune-mediated tissue damage in cases of
organ transplants for anti-aging, stress prevention and management and in the
improvement of overall health and quality of life.
ACKNOWLEDGEMENT
The authors are
grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global Inc. and
Trivedi Master Wellness for their support throughout the work.
CONFLICT OF INTEREST
Authors declare no
conflict of interest.
1.
Thomas KJ, Nicholl JP, Coleman P (2001) Use and
expenditure on complementary medicine in England: A population based survey.
Complement Ther Med 9: 2-11.
2.
Manya K, Champion B, Dunning T (2012) The use of
complementary and alternative medicine among people living with diabetes in
Sydney. BMC Complement Altern Med 12: 2-10.
3.
Barnes PM, Bloom B, Nahin R (2007) Complementary and
alternative medicine use among adults and children. United States, CDC National
Health Statistics Report # 12. 2008. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr012.pdf
4.
Astin JA, Pelletier KR, Marie A, Haskell WL (2000)
Complementary and alternative medicine use among elderly persons: One year
analysis of a blue shield Medicare supplement. J Gerontol A Biol Sci Med Sci
55: M4-M9.
5.
Müller O, Krawinkel M (2005) Malnutrition and health
in developing countries. Can Med Assoc J 173: 279-286.
6.
Li P, Zheng Y, Chen X (2017) Drugs for autoimmune
inflammatory diseases: From small molecule compounds to anti-TNF biologics.
Front Pharmacol 8: 460.
7.
Gautam SC, Gao X, Dulchavsky S (2007) Immunomodulation
by curcumin. Adv Exp Med Biol 595: 321-41.
8.
Lukác N, Massányi P (2007) Effects of trace elements
on the immune system. Epidemiol Mikrobiol Imunol 56: 3-9.
9.
Galland L (1988) Magnesium and immune function: An
overview. Magnesium 7: 290‐299.
10.
Frass M, Strassl RP, Friehs H, Mullner M, Kundi M, et
al. (2012) Use and acceptance of complementary and alternative medicine among
the general population and medical personnel: A systematic review. Ochsner J
12: 45-56.
11.
Trivedi MK, Mohan TRR (2016) Biofield energy signals,
energy transmission and neutrinos. Am J Modern Phys 5: 172-176.
12.
Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC,
et al. (2015) Antimicrobial sensitivity, biochemical characteristics and
biotyping of Staphylococcus saprophyticus:
An impact of biofield energy treatment. J Womens Health Care 4: 271.
13.
Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S
(2015) In vitro evaluation of
biofield treatment on Enterobacter
cloacae: Impact on antimicrobial susceptibility and biotype. J Bacteriol
Parasitol 6: 241.
14.
Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S
(2015) Evaluation of biofield modality on viral load of Hepatitis B and C
Viruses. J Antivir Antiretrovir 7: 83-88.
15.
Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC
et al. (2015) Antibiogram of biofield-treated Shigella boydii: Global burden of infections. Sci J Clin Med 4:
121-126.
16.
Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC,
et al. (2015) Evaluation of antibiogram, genotype and phylogenetic analysis of
biofield treated Nocardia otitidis.
Biol Syst Open Access 4: 143.
17.
Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S,
et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen.
J Clin Med Genom 3: 129.
18.
Peoples JJ, Trivedi MK, Branton A, Trivedi D, Nayak G,
et al. (2017) Skin rejuvenating effect of consciousness energy healing
treatment based herbomineral formulation. Am J Plant Biol 2: 77-87.
19.
Smith DM, Trivedi MK, Branton A, Trivedi D, Nayak G,
et al. (2017) Skin protective activity of consciousness energy healing
treatment based herbomineral formulation. J Food Nutr Sci 5: 86-95.
20.
Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M,
et al. (2015) Analysis of genetic diversity using simple sequence repeat (SSR)
markers and growth regulator response in biofield treated cotton (Gossypium hirsutum L.). Am J Agric
Forestry 3: 216-221.
21.
Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M,
et al. (2015) Evaluation of vegetative growth parameters in biofield treated
bottle gourd (Lagenaria siceraria)
and okra (Abelmoschus esculentus).
Int J Nutr Food Sci 4: 688-694.
22.
Trivedi MK, Branton A, Trivedi D, Nayak G, Balmer AJ,
et al. (2016) Evaluation of pro-inflammatory cytokines expression in mouse
splenocytes after incubation with biofield treated herbomineral formulation:
Effect of biofield energy healing treatment - The Trivedi Effect®.
Am J Biomed Life Sci 4: 87-97.
23.
Trivedi MK, Branton A, Trivedi D, Nayak G, Ellis MP et
al. (2016) Evaluation of pro-inflammatory cytokines expression in mouse
splenocytes after co-incubation with the biofield energy treated formulation:
Impact of the Trivedi Effect®. Int J Biomed Sci Eng 4: 40-49.
24.
Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S
(2015) Spectroscopic characterization of biofield treated metronidazole and
tinidazole. Med Chem 5: 340-344.
25.
Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S
(2015) Effect of biofield treatment on spectral properties of paracetamol and
piroxicam. Chem Sci J 6: 98.
26.
Trivedi MK, Tallapragada RM, Branton A, Trivedi D,
Nayak G, et al. (2015) Evaluation of atomic, physical and thermal properties of
bismuth oxide powder: An impact of biofield energy treatment. Am J Nano Res
Appl 3: 94-98.
27.
Trivedi MK, Patil S, Nayak G, Jana S, Latiyal O (2015)
Influence of biofield treatment on physical, structural and spectral properties
of boron nitride. J Material Sci Eng 4: 181.
28.
Tietez F (1969) Enzymic method for quantitative
determination of nanogram amounts of total and oxidized glutathione:
Applications to mammalian blood and other tissues. Anal Biochem 27: 502-502.
29.
Chiu DT, Stults FH, Tappel AL (1976). Purification and
properties of rat lung soluble glutathione peroxidase. Biochim Biophys Act 445:
558-558.
30.
Pulli B, Ali M, Forghani R, Schob S, Hsieh KLC et al.
(2013) Measuring myeloperoxidase activity in biological samples. PLoS One 8:
e67976.
31.
Feldman BF, Zinkl JG, Jain VC (2000) Laboratory
techniques for avian hematology. In: Schalm’s Veterinary Hematology. 5th
Edn. Lippincott Williams & Wilkins, Toronto, Canada.
32.
OECD (1992) OECD Guideline for Testing of Chemicals.
Organization for Economic Cooperation and Development, Paris, France.
33.
Karp SM, Koch TR (2006) Oxidative stress and
antioxidants in inflammatory bowel disease. Dis Mon 52: 199-207
34.
Qayyum R, Kurbanova N, Zia R, Adomaityte J (2014)
Serum selenium levels are associated with blood platelet count in US Adults.
Meeting: SHM Annual Meeting.
35.
Liu L, Li N, Lei T, Li K, Zhang Y (2014) The in vitro biological properties of
Mg-Zn-Sr alloy and superiority for preparation of biodegradable intestinal
anastomosis rings. Med Sci Monit 20: 1056-1066.
36.
Yang YS, Su YF, Yang HW, Lee YH, Chou JI et al. (2014)
Lipid-lowering effects of curcumin in patients with metabolic syndrome: A
randomized, double-blind, placebo-controlled trial. Phytother Res PTR 28:
1770-1777.
37.
Bunglavan SJ, Garg AK, Dass RS, Shrivastava S (2014)
Effect of supplementation of different levels of selenium as
nanoparticles/sodium selenite on blood biochemical profile and humoral immunity
in male wistar rats. Vet World 7: 1075-1081.
38.
Fox C, Ramsoomair D, Carter C (2001) Magnesium: Its
proven and potential clinical significance. South Med J 94: 1195-1201.
39.
Payahoo L, Ostadrahimi A, Mobasseri M, Khaje Bishak Y,
Farrin N et al. (2013) Effects of zinc supplementation on the anthropometric
measurements, lipid profiles and fasting blood glucose in the healthy obese
adults. Adv Pharm Bull 3: 161-165.
40.
Giannini EG, Testa R, Savarino V (2005) Liver enzyme
alteration: A guide for clinicians. CMAJ 172: 367-379.
41.
Srinivasan K, Sambaiah K (1991) The effect of spices
on cholesterol 7 alpha-hydroxylase activity and on serum and hepatic
cholesterol levels in the rat. Int J VitamNutr Res 61: 364-369.
42.
Madrigal-Santillán E, Madrigal-Bujaidar E,
Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J, et al. (2014)
Review of natural products with hepatoprotective effects. World J Gastroenterol
20: 14787-14804.
43.
Chanda S, Parekh J, Vaghasiya Y, Dave R, Baravalia Y
et al. (2015) Medicinal plants - From traditional use to toxicity assessment.
Int J Pharm Sci Res 6: 2652-2670.
44.
Amresh GR, Singh PN, Rao CV (2008) Toxicological
screening of traditional medicine Laghupatha (Cissampelos pareira) in experimental animals. J Ethnopharmacol 116:
454-460.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Pathology and Toxicology Research
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)